Soft polymer layers
During the past decades, major progress was achieved in the characterization of charging and structure of multi-responsive soft polymer layers by combined analysis of streaming current and surface conductivity data applying recent theories for the electrohydrodynamics of soft diffuse planar interphases. Here, I will provide a collection of key studies that demonstrate the application of the methodology for deciphering the mechanisms governing the charging of soft planar films and the impact of the charge on the polymer structure that may vary with the pH, salt concentration, or temperature of the system.
Electrokinetics of Poly(N-Isopropylacrylamide-co-Carboxyacrylamide) Soft Thin-Film. Evidence for Diffuse Segment Distribution in Swollen State.
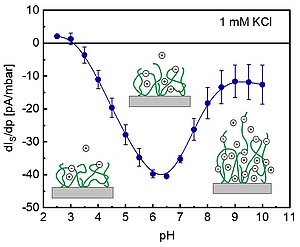
| Stimuli-responsive materials, as engineered from thermo-, light- or pH-sensitive polymers, have gained widespread interest in numerous scientific areas and industrial applications, e.g. biosensing, bioadhesion, drug delivery, tissue engineering, and microfluidics. A mandatory prerequisite for optimizing the performance of these materials is the complete analysis of the interfacial properties of their constitutive polymer films. In this study, we provide a quantitative interpretation of streaming current and surface conductivity measurements at poly(N-isopropylacrylamide-co-carboxyacrylamide) (PNiPAAM-co-CarboxyAAM) soft thin films. Date collected over a broad range of pH and salt concentration (pH 2.5-10, 0.1-10 mM KCl) at constant temperature of 22°C could be consistently interpreted applying the theory derived for electrokinetics of charged diffuse soft thin films complemented a general expression for the surface conductivity of such systems. In particular, the maximum in streaming current versus pH (left) was unambiguously attributed to the presence of an interfacial gradient in polymer segment density following heterogeneous expansion of the chains within the film upon swelling when increasing pH. |
| Reprinted with permission from Zimmermann et al. Langmuir 2010, 26, 18169, doi: https://doi.org/10.1021/la103526b. Copyright 2010 American Chemical Society. |
Electrokinetic Analysis to Reveal Composition and Structure of Biohybrid Hydrogels
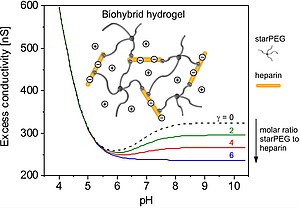
| Biohybrid hydrogels combining electrically neutral synthetic polymers and highly anionic glycosaminoglycans (GAGs) offer exciting options for regenerative therapies as they allow for the electrostatic conjugation of various growth factors. Unraveling details of ionization and structure within such networks defines an important analytical challenge that requires the extension of current methodologies. Here we present a mean-field approach to quantify the density of ionizable groups, GAG concentration, and cross-linking degree of such hydrogels based on experimental data from microslit electrokinetics and ellipsometry. An exemplary poly(ethylene glycol)-heparin system was analyzed to demonstrate how electrostatic fingerprints of hydrogels obtained by the introduced strategy can sensitively display composition and structure of the polymer networks.. |
| Reprinted with permission from Zimmermann et al. Anal. Chem. 2012, 84, 2995, doi: https://doi.org/10.1021/ac302538j. Copyright 2012 American Chemical Society. |
Evidence of Ion-Pairing in Cationic Brushes from Evaluation of Brush Charging and Structure by Electrokinetic and Surface Conductivity Analysis
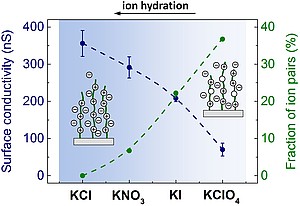
| Strong cationic poly(2-(methacryloyloxy)-ethyltrimethylammonium chloride) (PMETAC) brushes are widely employed as platform for studying fundamental physicochemical properties of permanently charged polymers at interfaces. This study provides detailed insights into the interfacial charging and structure PMETAC) brushes. Streaming current, surface conductivity, and swelling measurements performed at various pH values and salt concentrations were analyzed on the basis of the theory for electrohydrodynamics at diffuse soft interfaces to evaluate the density of ionized groups and the characteristic segment density distribution across the brush-electrolyte interface. Furthermore, we investigated the effects of chaotropic anions on the brush charge and on the magnitude of ion pairing from refined interpretation of surface conductivity data collected in 1 mM KNO3, KI, and KClO4 solutions. |
| Reprinted with permission from Zimmermann et al. J. Phys. Chem. C 2017, 121, 2915, doi: https://doi.org/10.1021/acs.jpcc.6b1253. Copyright 2017 American Chemical Society. |
