Charging by unsymmetrical water ion adsorption
Charging of solid-liquid interfaces is an ubiquitous phenomenon of particular importance in surface and materials science. On many solid surfaces the charging is caused by ionization of functional groups, e.g., carboxyl or amino groups. However, also for surfaces without ionisable surface groups charging is observed in aqueous environments. The phenomenon is often attributed to the unsymmetrical adsorption of hydroxide and hydronium ions. Despite of numerous theoretical and experimental studies, very little is known on the interaction of hydroxide and hydronium ions with water-hydrophobic interfaces. To address this question, we systematically perform electrokinetic measurements at various material surfaces.
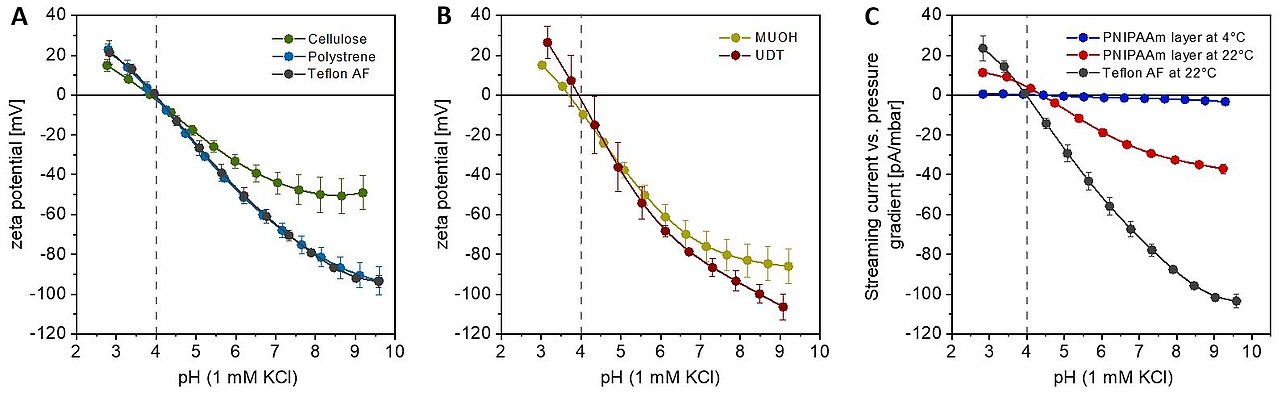
Zeta potential vs. solution pH of polymer films w/o ionizable surface groups in 1 mM KCl solution: (A) Cellulose, polystyrene, and Teflon® AF. (B) Self-assembled monolayers prepared from mercaptoundecanol (MUOH) and undecanthiol (UDT) on gold. (C) pH dependence of the streaming current versus pressure gradient for poly(N-isopropylacrylamide-co-N-(1-phenylethyl)) acrylamide (PNIPAAm) thermo-responsive hydrogel layers, immobilized on Teflon® AF coated substrates, at T = 22°C and at T = 4°C. Adapted from Current Opinion Colloid Interface Sci. 2010, 31, 9462-9472, Zimmermann et al. Hydroxide and hydronium ion adsorption - A survey. Copyright 2010, with permission from Elsevier.
References
[1] Zimmermann et al. Electrokinetic Measurements Reveal Interfacial Charge at Polymer Films Caused by Simple Electrolyte Ions. J. Phys. Chem. B 2001, 105, 8544-8549, doi: https://doi.org/10.1021/jp004051u
[2] Zimmermann et al. Hydroxide and hydronium ion adsorption - A survey. Current Opinion Colloid Interface Sci. 2010, 31, 9462-9472, doi: https://doi.org/10.1016/j.cocis.2010.01.002
